What are schedule 4 drugs, and why are they regulated differently from other substances? These drugs, such as some well-known anxiety and insomnia medications, have valid medical uses but are less likely to be abused than those in more restrictive schedules. Our guide will dissect the balance of therapeutic benefits and regulations designed to prevent misuse and dependency, providing you with both examples of these drugs and a comprehensive understanding of how they are controlled.
Key Takeaways
- Schedule IV drugs are classified as substances with a lower risk of abuse and dependence compared to Schedule I-III drugs but still carry a risk for misuse and psychological and physical dependence.
- The Controlled Substances Act (CSA) outlines the legal framework for drug scheduling and the DEA enforces it, ensuring adherence to regulations concerning manufacture, distribution, and dispensation of controlled substances.
- While Schedule IV drugs are deemed important in medical treatments for disorders such as anxiety, insomnia, and seizures, they must be prescribed and used with caution due to their potential for abuse, addiction, and side effects.
Schedule IV Drugs: Overview and Classification
Under the Controlled Substances Act, drugs are classified into five schedules. Schedule IV drugs, a crucial category among them, are known for their low potential for abuse and low risk of dependence. This classification is mainly determined by the drug’s acceptable medical use and its lower potential for abuse or dependency compared to Schedule I, II, and III drugs.
Indeed, compared to other schedules, Schedule IV drugs are distinct due to their reduced potential for abuse and dependence. But, it’s crucial to remember that despite the lower risk, these drugs can still lead to physical and psychological dependence.
Characteristics of Schedule IV Drugs
Generally, Schedule IV drugs are categorized as controlled substances, indicating a regulated status. These substances pose a lower risk of physical or psychological dependence compared to Schedule III drugs and those in Schedules I or II. This categorization takes into account the accepted medical use of these substances in treatments. However, it’s essential to remember that the potential for abuse and dependence, although lower, is not entirely absent.
The unique characteristic of Schedule IV drugs is their balance between efficacy and risk. They provide much-needed relief for several medical conditions while maintaining a lower potential for abuse. This characteristic makes them a preferred choice for healthcare providers in many instances. But, it also necessitates adherence to prescription guidelines to mitigate any potential risks.
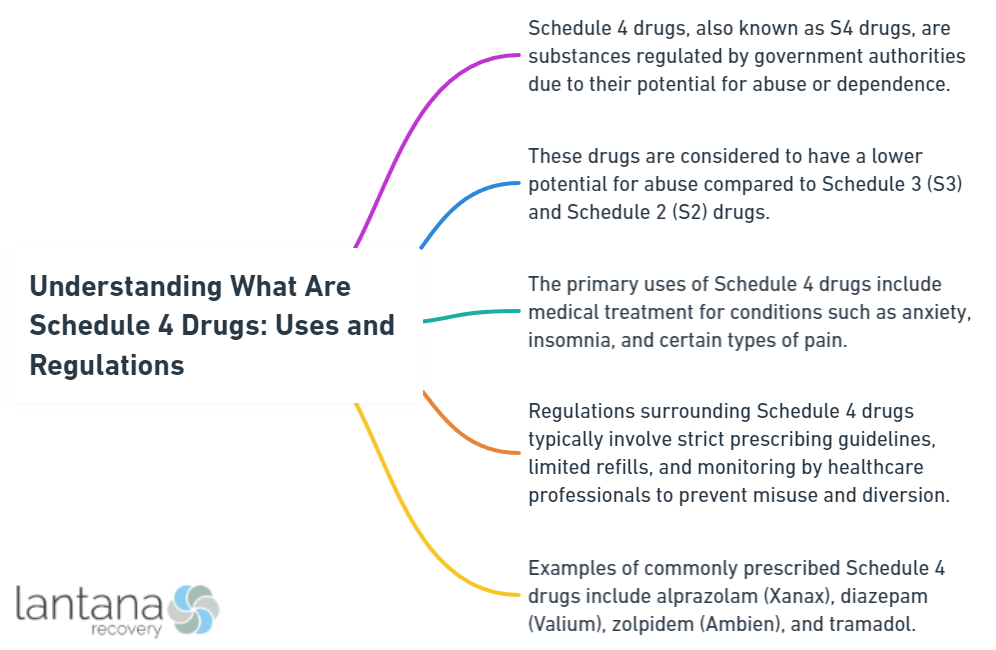
Examples of Schedule IV Drugs
Several drugs fall under the category of Schedule IV, including:
- Alprazolam, which is primarily prescribed for anxiety disorders
- Zolpidem, which is mainly for sleep disorders
- Lorazepam, which is used for anxiety disorders and as a sedative before medical procedures
- Diazepam, which is primarily prescribed for anxiety disorders
- Tramadol, which is an analgesic
These drugs are just the tip of the iceberg, with many more falling under the Schedule IV category, while Schedule II drugs represent another level of control.
Other examples include Ramelteon (Rozerem), a drug used to treat insomnia. Acting as a melatonin receptor agonist, it does not affect GABA receptors, which reduces its abuse potential. Doxepin (Silenor), another Schedule IV drug, serves as a sedative in the treatment of insomnia by acting on histamine (H1) receptors. These examples highlight the diverse range of Schedule IV drugs and their versatile uses in medical treatments.
Alcohol Rehab South Carolina
Alcohol rehab in South Carolina offers vital support and resources for individuals struggling with alcohol addiction. With a range of programs tailored to meet the diverse needs of patients, these facilities provide a structured environment conducive to recovery. In South Carolina, alcohol rehab centers employ a multidisciplinary approach, combining therapy, counseling, and medical intervention to address the physical, psychological, and emotional aspects of addiction. Through personalized treatment plans, individuals receive comprehensive care aimed at breaking the cycle of dependency and promoting long-term sobriety. Moreover, these rehab centers often integrate aftercare services to support individuals as they transition back into their communities, equipping them with the skills and strategies necessary to maintain a sober lifestyle beyond treatment.
The Controlled Substances Act and Drug Scheduling

The Controlled Substances Act (CSA), established in the 1970s, provides a legal framework for the regulation of drugs, including controlled substance, based on their potential for abuse and accepted medical use. Under the controlled substance act, substances are organized into five schedules, with Schedule I having no accepted medical use and high potential for abuse, and Schedule V having lower potential for abuse and accepted medical uses. This classification requires considering several factors, including the drug’s abuse potential, pharmacological effects, and patterns of abuse.
The CSA also allows for substances to be controlled, decontrolled, or rescheduled through a process that can be initiated by:
- the Drug Enforcement Administration (DEA)
- Health and Human Services (HHS)
- petition from interested parties such as drug manufacturers and medical associations
This flexibility ensures that the classifications remain relevant and accurate over time, reflecting the latest scientific research and societal trends.
Drug Enforcement Administration’s Role
The DEA plays a pivotal role in regulating the use and distribution of controlled substances. It is tasked with:
- Investigating major violations of controlled substances laws across interstate and international borders
- Preparing violators for prosecution
- Targeting assets stemming from or planned for use in illegal drug trafficking through asset seizure and forfeiture.
Enforcing regulations regarding the manufacture, distribution, and dispensation of legally produced controlled substances is a critical role of the DEA under the Controlled Substances Act. To ensure effective enforcement, the DEA collaborates with various law enforcement agencies at the federal, state, local, and foreign levels for joint drug enforcement initiatives. Internationally, the DEA works with organizations such as the United Nations and INTERPOL to help control drug distribution worldwide.
Medical Uses of Schedule IV Drugs
Now that we understand the classification and regulations of Schedule IV drugs, let’s delve into their currently accepted medical use. These drugs are prominently used in the treatment of anxiety disorders due to their therapeutic effects. They also play a vital role in managing insomnia, providing relief for individuals struggling with sleep disorders.
In addition to these, Schedule IV drugs are crucial in controlling seizure disorders, including epilepsy.
Anxiety Disorders
Anxiety disorders, characterized by excessive and persistent worry, are widespread mental health issues. A primary class of Schedule IV drugs prescribed for managing these disorders is benzodiazepines. Common benzodiazepines such as alprazolam (Xanax), clonazepam (Klonopin), and diazepam (Valium) are effective in the treatment of anxiety disorders.
Alprazolam, commonly known as Xanax, is frequently prescribed for generalized anxiety disorder (GAD) and panic disorder with or without agoraphobia. Another benzodiazepine, Lorazepam, available under the brand name Ativan, is used to treat anxiety disorders due to its calming effect on the central nervous system. These drugs, while providing relief from the symptoms of anxiety disorders, need to be used judiciously due to their potential for dependence.
Insomnia
Insomnia, a common sleep disorder, is another condition where Schedule IV drugs have proven efficacy. Drugs approved for the treatment of insomnia include benzodiazepines such as triazolam, estazolam, temazepam, quazepam, and flurazepam, as well as nonbenzodiazepine ‘Z drugs’ like zolpidem, zaleplon, and eszopiclone.
Nonbenzodiazepine sedatives like zolpidem (Ambien) are available in multiple formulations, including immediate and modified-release tablets, sublingual tablets, and oral spray. These different formulations cater to different needs regarding the onset of action and associated treatment costs.
Zaleplon (Sonata) offers a beneficial option for patients with middle-of-the-night awakenings due to its rapid onset and short duration of action, while eszopiclone (Lunesta) is suitable for the long-term treatment of insomnia, provided that the patient has sufficient time for sleep.
Seizure Disorders
Seizure disorders, including epilepsy, pose significant challenges for patients and healthcare providers alike. Schedule IV drugs, particularly benzodiazepines, are utilized in their treatment due to their anticonvulsant properties. Clonazepam (Klonopin) and diazepam (Valium) are known for their effectiveness in managing seizure disorders, including status epilepticus.
Phenobarbital, another Schedule IV drug, influences the GABA neurotransmitter system, making it effective in controlling seizures. Clonazepam (Klonopin) is specifically indicated for the treatment of certain types of seizure disorders, showcasing the focused use of some Schedule IV drugs in epilepsy care.
These examples underscore the significant contribution of Schedule IV drugs in managing challenging neurological conditions.
Is Addiction Genetic
The question of whether addiction is genetic has been a subject of intense study and debate in the field of psychology and neuroscience. Research indicates that genetic factors do play a significant role in predisposing individuals to addiction. Studies on twins and adopted children have shown that there is a higher likelihood of developing an addiction if a biological family member also struggles with substance abuse. However, it’s important to recognize that genetic predisposition is just one piece of the puzzle. Environmental factors, such as upbringing, peer influence, and access to substances, also play crucial roles in the development of addiction. Therefore, while genetics can increase susceptibility to addiction, it’s not a deterministic factor, and other influences must be considered in understanding and addressing addiction effectively.
Risks and Side Effects of Schedule IV Drugs

While Schedule IV drugs have significant therapeutic benefits, they also carry risks of drug’s abuse, addiction, and side effects. Despite their lower potential for abuse compared to Schedule I, II, or III substances, they still carry a significant risk of physical or psychological dependence. Overuse and misuse of these drugs can lead to addiction, overdose, and even death.
It’s vital to remember that while Schedule IV drugs are safer than drugs in higher schedules, they still need to be used with caution. Healthcare providers need to monitor their use closely due to their potential for misuse and dependence. Common side effects of Schedule IV drugs include:
- sedation
- dizziness
- weakness
- unsteadiness
- sleep disturbances
In the elderly, the use of anabolic steroids can impair cognitive function and increase the risk of dementia and dementia-like illnesses.
Potential for Abuse and Addiction
Prolonged or inappropriate use of Schedule IV drugs can lead to substance use disorder. Benzodiazepines and other Schedule IV drugs can lead to serious psychological and physical dependence, evidenced by withdrawal symptoms similar to those of alcohol withdrawal and a need for increasing dosages due to tolerance. Complex withdrawal syndromes that require medical management are a concern with these drugs, particularly benzodiazepines, and withdrawal symptoms can occur even with gradual tapering.
Some common withdrawal symptoms include:
- Anxiety
- Insomnia
- Tremors
- Sweating
- Nausea
- Headaches
- Irritability
If you are experiencing withdrawal symptoms or are concerned about your use of Schedule IV drugs, it is important to seek medical help and support.
The risk of developing substance use disorder with Schedule IV drugs is heightened with prolonged or inappropriate use, higher doses, non-medical reasons, and certain populations such as those with a history of substance abuse or mental health disorders. Even overdose of these drugs can have severe side effects. For instance, tramadol overdose can lead to difficulty breathing, slow or irregular heartbeat, seizure, and possible loss of consciousness, highlighting the dangers of Schedule IV drug overdose and misuse.
Common Side Effects
While the therapeutic effects of Schedule IV drugs are undeniable, their side effects cannot be overlooked. Some common side effects of benzodiazepines include:
- Drowsiness
- Confusion
- Impaired coordination
- Increased anxiety
- Aggression
- Insomnia
- Aggressive behavior
- Expressive anger
These side effects can be particularly risky for the elderly.
Sedative-hypnotics can result in side effects such as daytime drowsiness, amnesia, and lethargy, that may interfere with activities requiring mental alertness. Tramadol, another Schedule IV drug, commonly leads to dizziness, nausea, constipation, headache, and somnolence, affecting tasks like driving or operating machinery. These side effects underscore the importance of using Schedule IV drugs judiciously and under the guidance of a healthcare provider.
Prescription Guidelines and Regulations for Schedule IV Drugs

The prescription of Schedule IV drugs, which are a category of prescription drugs, is strictly regulated to ensure their safe use and prevent misuse and addiction. Healthcare providers are responsible for:
- Assessing and treating patients’ pain appropriately, including treating chronic pain
- Avoiding addiction by initiating therapy with the lowest effective dose
- Monitoring for both benefits and risks of the therapy
Prescription Requirements
Schedule IV drug prescriptions must adhere to specific requirements. They can be prescribed via:
- A signed paper prescription
- A faxed signed paper prescription
- Electronic prescriptions that comply with regulations
- Oral prescriptions that are promptly transcribed to written form
All prescriptions must include all requisite details aside from the prescriber’s signature.
Prescriptions for Schedule IV substances are not valid if filled or refilled more than six months after the prescription date, and no more than five refills are allowed for such prescriptions. Oral authorizations for refills of Schedule IV drugs are permitted provided that the combined quantity of refills does not surpass five and does not extend beyond six months from the initial prescription date. Pharmacists are required to document all refills of Schedule IV substances, noting the controlled substance’s name and dosage form, fill date, dispensed quantity, dispensing pharmacist’s initials, and the total refills issued.
Monitoring and Compliance
Monitoring and compliance measures are in place to ensure the safe use of Schedule IV drugs. Pharmacies are permitted to transfer prescription information for Schedule IV drugs for refill purposes only once unless they share a real-time, online database. The transferred prescription information must be communicated between licensed pharmacists and include details like original dispensing date, remaining refills, and the transferring pharmacist’s name.
Retail and central fill pharmacies must maintain accurate records of transmittal, dispensing, and delivery for Schedule IV drugs; all related prescriptions must be retained for two years from the last refill date. EPCS regulations mandate the use of EPCS-certified applications for electronic prescriptions of controlled substances, including exemptions for certain prescribers and emergency situations.
Prescription Drug Monitoring Programs (PDMPs) collect data that assists healthcare providers in monitoring prescription use and identifying potential abuse, aiding in more informed clinical decisions.
What Are Schedule 8 Drugs?
Schedule 8 drugs, often referred to as controlled substances, are medications and substances categorized by regulatory bodies as having a high potential for abuse or addiction, as well as severe adverse effects. These substances are tightly regulated due to their significant risks to public health and safety. Examples of Schedule 8 drugs include strong opioids like morphine, fentanyl, and oxycodone, as well as certain stimulants and sedatives. Their distribution and use are strictly controlled by law, typically requiring special licenses and strict prescribing guidelines to minimize misuse and diversion. Additionally, healthcare professionals must exercise caution and adhere strictly to prescribing protocols when dealing with Schedule 8 drugs to ensure patient safety and prevent illicit use.
Safe Use and Disposal of Schedule IV Drugs

While we’ve discussed the uses, risks, and regulations of Schedule IV drugs, it’s also crucial to understand their safe use and disposal. The FDA provides a ‘flush list’ for medications that have a high potential for misuse or could be extremely harmful if accidentally ingested, which allows for certain drugs to be flushed down the toilet as a means of disposal.
Local drug ‘take back’ programs and events are periodically offered to provide a safe and secure method for disposing of unused or expired medications, including Schedule IV drugs, ensuring the drug’s acceptable medical use is not compromised.
Proper Storage
Secure storage of Schedule IV drugs is essential to prevent unauthorized access and misuse. These drugs should be stored securely to limit the risk of unauthorized access and usage. Proper storage practices are crucial for preventing unauthorized access to Schedule IV drugs.
Secure storage of Schedule IV drugs is essential in mitigating the potential for misuse and diversion. While specific secure storage practices for Schedule IV drugs have not been identified in the available research, it’s safe to assume that these drugs should be kept out of reach of children and in a secure location where they cannot be accessed by unauthorized individuals.
Disposal Methods
When it comes to disposal, there are several environmentally responsible methods for disposing of unused or expired medications. Local collection programs offer an environmentally responsible method for the disposal of unused or expired medications. The FDA recommends against flushing most unused medicines down the sink or toilet to minimize environmental impact, with the exception of those on the ‘flush list’ due to their high risk if misused.
Most medications can be disposed of in household trash after being mixed with undesirable substances and placed in a sealable bag, ensuring they are taken to a regulated disposal site. This method of disposal is accessible, easy, and ensures that unused medications are safely discarded, preventing potential misuse and environmental contamination.
Summary
In this journey through the realm of Schedule IV drugs, we’ve explored their classification, medical uses, potential risks, regulations, and safe usage and disposal methods. These drugs, with their low potential for abuse and accepted medical uses, play a crucial role in treating various health conditions such as anxiety disorders, insomnia, and seizure disorders. However, they also carry risks of physical and psychological dependence, addiction, and side effects, underscoring the need for stringent regulations and careful monitoring.
Understanding Schedule IV drugs, their uses, risks, and regulations is not only essential for healthcare professionals but also for the general public. Increased awareness can lead to informed health decisions, prevent misuse, and ultimately contribute to a healthier society. So, let’s continue to learn, stay informed, and make conscious health choices.
Frequently Asked Questions
Why is Xanax schedule 4?
Xanax is classified as Schedule IV under the Controlled Substances Act due to its medical uses for anxiety and panic disorder, as well as its potential for misuse and addiction.
Is caffeine a Schedule 4 drug?
No, caffeine is not classified as a Schedule 4 drug and is not considered a controlled substance. It does not currently fall under any of the controlled substance schedules (Standard response).
Is Gabapentin a Schedule 4 drug?
Yes, gabapentin is classified as a Schedule V controlled substance in some states, while it is controlled federally in all states due to its similarity to pregabalin, which is a Schedule V drug. Therefore, it is considered a Schedule 5 controlled substance in some states.
What is a Schedule 4 controlled drug?
A Schedule 4 controlled drug, such as Xanax or Soma, has a low potential for abuse and a low risk of dependence, making it less dangerous than other controlled substances.
What are some examples of Schedule IV drugs?
Schedule IV drugs include Alprazolam, Zolpidem, Lorazepam, Diazepam, and Tramadol. These medications are commonly prescribed for different medical conditions.









